Abstract
Background: Multiple myeloma (MM) remains incurable; despite progress in improving outcomes with novel therapeutics, nearly all patients (pts) will experience relapse. Managing relapsed/refractory MM (RRMM) remains challenging, given the multitude of combination regimens incorporating multiple drug classes, the continued development of new agents, uncertainty in treatment (Tx) sequencing in later lines of therapy, and the heterogeneity of MM. Therefore, we evaluated the real-world (RW) variations in Tx patterns and clinical outcomes in a US population of pts with RRMM receiving ≥3 lines of therapy (LOT).
Methods: This retrospective observational study used de-identified electronic health records data from the Concert AI (CAI) Patient360 database, which represents primarily community-based oncology centers across the US. Data were included from adults with MM (diagnosed between January 1, 2011 and March 30, 2022), no other primary cancer diagnosis (Dx), ≥3 LOT, ≥1 R/R event (International Multiple Myeloma Working Group criteria) before the third-line (3L), and ≥2 mo follow-up.
Proprietary progression-based LOT algorithms were used. Conditions for LOT advancement included a change in regimen after 30 d of the start of the prior regimen (ie, combination rule); and discontinuation of a drug following a relapse or progression event. Prespecified allowable drug switches (eg, dexamethasone for prednisone) did not constitute LOT advancement. For regimens starting ≤30 days before a transplant event, the combination rule was shortened to the transplant date; the regimen only included drugs administered up to that point. Transplant itself was not considered a regimen.
Median RW time to discontinuation (rwTTD), progression-free survival (rwPFS), and overall survival (rwOS) by lines were calculated via Kaplan-Meier estimates. In cases of no observed progression or death events, pts who had a subsequent LOT were censored 1 day before the next LOT starting date; pts without a subsequent LOT were censored at either the last active date or the end of study period, whichever was earlier.
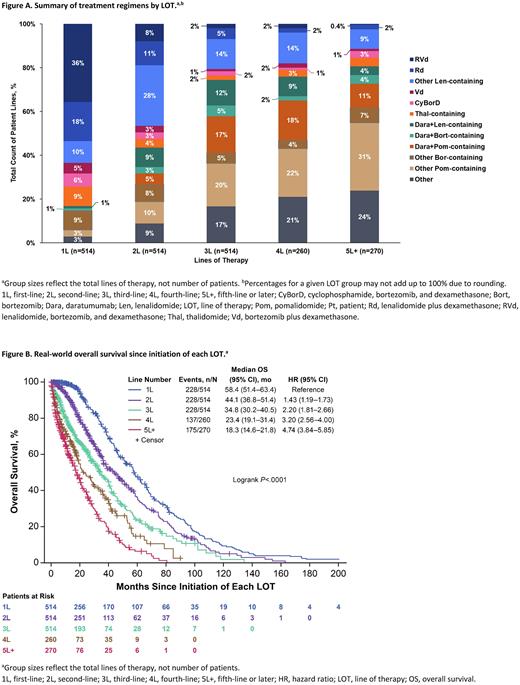
Results: Overall, 514 pts who initiated 3L or later therapy and fulfilled eligibility criteria were included. Of these, 254 had 3 LOT, 137 had 4 LOT, and 123 had ≥5 LOT. Median age at Dx was 63.8 y (3 LOT), 64.6 y (4 LOT), and 59.8 y (≥5 LOT; P=.0016). Most pts were treated in community centers (74% [3 LOT], 66% [4 LOT], and 75% [≥5 LOT; P=.1234]). The most frequent disease stage at Dx was stage III for each LOT group: 33% (3 LOT), 31% (4 LOT), and 41% (≥5 LOT; P=.5325); stage data were not available for 21%, 18%, and 18%, respectively. Median follow-up since Dx was 57.2 mo (3 LOT), 63.8 mo (4 LOT), and 91.1 mo (≥5 LOT; P<.0001). There were 260 fourth-line (4L) regimens and 270 fifth-line or later (5L+) regimens (Figure A). Lenalidomide + bortezomib ± dexamethasone (RVd) was the most common 1L regimen (36%), with use markedly decreasing in later LOT. The most common 2L regimens were Len-containing (excluding RVd and Rd; 28%). Pomalidomide (Pom)-containing regimens (excluding daratumumab [Dara] + Pom) were the most common 3L (20%), 4L (22%), and 5L+ (31%) regimens. Dara + Pom-based regimens comprised 17%, 18%, and 11% of 3L, 4L, and 5L+ regimens, compared with 0% and 5% of 1L and 2L.
Median (95% CI) rwTTD from 1L, 2L, 3L, 4L, and 5L+ was 7.7 mo (6.9−8.2 mo), 10.5 mo (9.5−11.8 mo), 10.0 mo (8.8−11.0 mo), 7.1 mo (6.3−8.5 mo), and 5.6 mo (4.4−6.3 mo). Median (95% CI) rwPFS differed across LOT: 23.2 mo (20.2−26.5 mo) during 1L, 13.1 mo (11.9−14.3 mo) during 2L, 15.0 mo (11.8−18.3 mo) during 3L, 13.6 mo (8.9−16.7 mo) during 4L, and 6.7 mo (4.3−8.8 mo) during 5L+. Median rwOS (95% CI) also differed: 58.4 mo (51.4−63.4 mo) from 1L, 44.1 mo (36.8−51.4 mo) from 2L, 34.8 mo (30.2−40.5 mo) from 3L, 23.4 mo (19.1−31.4 mo) from 4L, and 18.3 mo (14.6−21.8 mo) from 5L+ (Figure B). All P-values from the log-rank tests were <.001.
Conclusion: This RW analysis demonstrated increasing Tx heterogeneity from 1L to 5L+, underlining the Tx uncertainty among physicians managing pts with MM and later LOT. RVd and other Len-containing regimens were the most common 1L and 2L therapies. In 3L, 4L, and 5L+, Pom-containing regimens were most common. The results may have been impacted by emerging MM Tx options since 2011. Overall, outcomes, including rwOS, were worse with later LOT in pts with RRMM compared with earlier LOT. These findings highlight the need for effective therapies for pts with RRMM and ≥3 LOT.
Disclosures
Girvan:Eli Lilly: Current equity holder in publicly-traded company, Ended employment in the past 24 months; AbbVie: Current Employment, Current equity holder in publicly-traded company. Yu:Guardant Health: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months; AbbVie: Current Employment, Current equity holder in publicly-traded company. Kamalakar:AbbVie: Current Employment, Current equity holder in publicly-traded company. Mearns:Genentech: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Roche: Current equity holder in publicly-traded company. Nixon:Roche / Genentech: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Cornell:AbbVie: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal